Nuclear Physics
The nucleus
Radioactivity
Applications
Particle accelerators
© The scientific sentence. 2010
|
Nuclear Physics &
Particle Physics
Particle accelerators
Scintigraphy
Gamma camera
Photomultiplier tube (PMT)
1. Gamma camera
The radiotracer, injected into a vein, emits gamma
radiation as it decays. A gamma camera scans
the radiation area and creates an image.

A gamma camera is an imaging device used in nuclear scanning.
I was invented by H. Anger in the 1960s and is often referred to as the
Anger camera.
A gamma camera, also called a scintillation camera creates images of the
gamma radiation emitting radioisotopes from the human body part to scan. It is
a technique known as scintigraphy .
The applications of scintigraphy include drug development and nuclear medical
imaging to view and analyse images of the human body or the distribution
of injected, inhaled, or ingested radionuclides emitting gamma rays.
2. Gamma camera principle
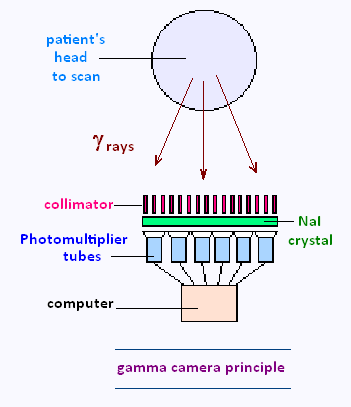
In clinical practice, an Anger camera consists of a collimator, placed between
the patient and the detector surface made of crystal NaI(Ti), Sodium iodide with
Thallium as a dopant.
The collimator is made out of a highly absorbing material such as lead, serving
to suppress gamma rays that deviate substantially from the vertical. The simplest
collimators contain parallel holes.
A phototube or photomultiplier is placed between a
computer and the crystal.
The information is recorded onto film as an analogue
image or onto a computer, coupled to the camera, in digital form.
3. Sodium Iodide scintillation detector
Sodium Iodide is a type of detector among the scintillation detectors.
Gamma rays penetrate into the NaI crystal where they may experience
interactions with the atoms inside the crystal.
These interactions release photons of visible light from the atom and
travel short distances within the crystal.
Thallium (Tl) is used as a dopant for NaI because to increase its scintillation
efficiency, that is the number of visible photons being output by the crystal.
It also helps to improve the crystal's transparency to those emitted photons,
that will be measured by equipment outside the crystal.
These photons of visible light are detected by a photocathode to
produce an electrical signal, which is then amplified and processed to
create a gamma ray spectrum.
4. Photomultiplier tube (PMT)

A photomultiplier tube (PMT) is an extremely
sensitive photocell used to convert light signals of a few hundred photons
into a usable current pulse.
A PMT consists of two major elements: a photocathode coupled to an
electron multiplier; that are contained within an evacuated glass envelope.
The photocathode comprises a photosensitive coating, made of alkali metals. Light
photons liberate low-energy electrons (1 eV or less) from the photocathode.
Because the number of photoelectrons produced is about the same as the number of
incident light photons, the photoelectrons total charge will be too
small to provide a detectable electrical signal. Hence the need for an
electron multiplier.
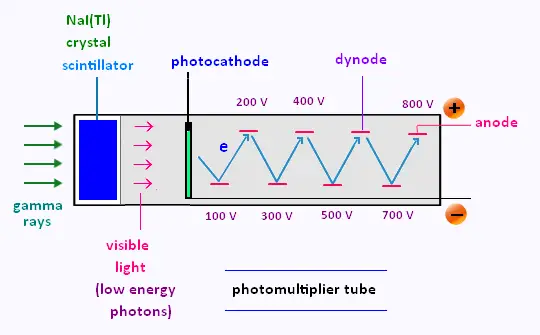
The electron multiplier consists of an arrangement of many dynodes.
The dynodes are intermediate electrodes located between the photocathode and the anode,
also called the collector.
Each photoelectron is accelerated towards and then strikes the first dynode, which has
a special surface. Here its kinetic energy is absorbed and results in the emission of
several secondary electrons.
This process of electron multiplication continues down the dynode chain, until
eventually a large signal is collected at the anode. Generally, each electron that
strikes a dynode will produce about four secondary electrons, depending on the applied
voltage and the number of dynodes.
That is, if one electron is released from the photocathode, a phototube with n dynodes
will deliver 4n, that is about a million electrons to the collector.
The electron multiplier serves both as a collector of photoelectrons and as an
amplifier that greatly increases the number of electrons.
After amplification, a typical scintillation pulse will give rise to 1millions
electrons, sufficient to generate a charge signal that can be collected at the anode.
|
|