Quantum Mechanics
Schrodinger equation
Quantum Mechanics
Propagators : Pg
Quantum Simple Harmonic
Oscillator QSHO
Quantum Mechanics
Simulation With GNU Octave
© The scientific sentence. 2010
|
| Many-electron atoms: Helium atom
Let's recall:
The Schrodinger time-independent equation:
- (ℏ 2/2m)∂2ψ(r)/∂r2 + V(r) ψ(r) = E ψ(r) (1)
1. The Helium atom example:
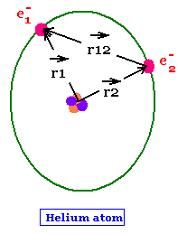
The helium atom has two electrons. Its nucleus has two protons and two neutrons, then a nuclear charge of Z = + 2 e, where e is the negative charge of the electron.
Using Schrodinger's equation, we want to find
the wave functions and the energies of the two electrons.
The Hamiltonian of the atom is:
H = - (ħ2/2m) ∇12
- 2 e2/r1
- (ħ2/2m) ∇22
- 2 e2/r2
+ e2/r12
• (- ħ2/2m) ∇12
represents the kinetic energy of the first electron,
• (- ħ2/2m) ∇22
the kinetic energy of the second electron,
• - 2 e2/r1
the electrostatic attraction between the nucleus
and the first electron,
• - 2 e2/r2
the electrostatic attraction between the nucleus and the
second electron, and
• + e2/r12
the electrostatic repulsion between the two electrons,
respectively.
The last term + e2/r12 is the term that make
the problem more complicated.
In this lecture, we will test three approximations and gives their
corresponding results for the Helium atom at its ground-state.
In the first step, we will neglect it, in the
second step, we will use the variation principle to correct the energy
found using the first step, and in the last step, we will use the effective charge to obtain a result more closer to one of obtained by experiment for the ground state: 78.98 eV.
2. Solutions for Helium without
electrostatic repulsion term + e2/r12:
If we neglect the electrostatic repulsion term, we will have:
H = H1 + H2, where:
H1 = - (ħ2/2m) ∇12
- 2 e2/r1 , and
H2 = - (ħ2/2m) ∇22
- 2 e2/r2
The Hamiltonian becomes the sum of separate Hamiltonians
for each electron., and the wave-function of the atom ψ (r1, r2)
becomes separable, and can be written as:
ψ (r1, r2) = ψ1 (r1) ψ2 (r2)
Schrodinger equation:
Hψ (r1, r2) = E ψ (r1, r2) becomes also separable as:
H1 ψ1 (r1) = E1 ψ1 (r1) , and
H2 ψ2 (r2) = E2 ψ2( r2) ,
with E = E1 + E2
The two above Schrodinger equations are just the hydrogen-like
ones, where we can replace Z by 2, and obtain for both electrons
in their ground states.
With
ao = ħ2/m e2, and
Eo = - m e4/2ħ2 =
- e2/2ao = -13.6 eV
e2 = q2/4πεo.
For the wave functions:
ψ1 (r1) = 4/[2π ao3]1/2
exp {- 2 r1/ao}
ψ2 (r2) = 4/[2π ao3]1/2
exp {- 2 r2/ao}
For the ground-state energies:
E1 = E2 = 4 Eo = - 4 x 13.6 = - 54.4 eV
Therefore:
E = E1 + E2 = 8 Eo = - 54.4 - 54.4 = - 108.8 eV
We still are far from the value obtained by experiment for the
ground state which is 78.98 eV.
Therefore, the neglected electron-electron repulsion term
must make a large contribution to the helium ground-state energy.
Let's use now the variational principle.
3. Solutions for Helium with
electrostatic repulsion term + e2/r12:
Let's rewrite the atomic wave function:
ψ (r1, r2) = ψ1 (r1) ψ2 (r2) =
4/[2π ao3]1/2
exp {- 2 r1/ao}
4/[2π ao3]1/2
exp {- 2 r2/ao} =
8/[π ao3]
exp {- 2 (r1 + r2)/ao}
The term to add to the total energy E is <U12>, where
< U12 > is the expectation value related to the repulsion term
+ e2/r12, since the expectation value of the Hamiltonian
is <H> = E + <U12>.
<U12> = <ψ(r1,r2)|e2/r12|ψ(r1,r2)>
= <ψ(r1,r2)|[e2/|r1 - r2|]|ψ(r1,r2)>
= e2 as∫ |ψ(r1,r2)|2 [1/|r1 - r2|] dr13 dr23
With:
ψ (r1, r2) = 8/[π ao3]
exp {- 2 (r1 + r2)/ao}, we have:
<U12> = e2 {8/[π ao3]} 2
∫ ∫ exp {- 4 (r1 + r2)/ao} [1/|r1 - r2|] dr13 dr23
With:
R1 = 2r1/ao, R2 = 2r2/ao,
As the volume element dr3 = r2 dr, we have similarly:
dr13 = r12 dr1, and
dr23 = r22 dr2
We have then :
dR13 = R12 dR1 = (2r1/ao)2 dR1 =
R12 (2/ao) dr1 = 4(r12/ao2) (2/ao) dr1
= (8/ao3)dr13.
Therefore :
dr13 = (ao3/8)dR13
So
dr13 = (ao3/8)dR13
dr23 = (ao3/8)dR23
we have:
<U12> = e2 {8/[π ao3]} 2
∫ ∫ exp {- 2 (R1 + R2)} [1/|R1 - R2|] dR13 dR23 (2/ao) (ao3/8) (ao3/8) =
= (e2/π2) (2/ao)∫ ∫ exp {- 2 (R1 + R2)} [1/|R1 - R2|] dR13 dR23
Since Eo = - e2/2ao = -13.6 eV , we have then :
<U12> =
- (4 Eo/π2) ∫ ∫ exp {- 2 (R1 + R2)} [1/|R1 - R2|] dR13 dR23
With:
R1 - R2 = [R12 + R22 - 2R1 R2 cos θ]1/2
Where θ is the angle between R1 and R1, we have:
<U12> =
- (4 Eo/π2)
∫ ∫ exp {- 2 (R1 + R2)} [1/([R12 + R22 - 2R1 R2 cos θ]1/2)] dR13 dR23
<U12> =
- (4 Eo/π2)
∫ ∫ exp {- 2 (R1 + R2)} [1/([R12 + R22 - 2R1 R2 cos θ]1/2)] dR13 dR23
The steps for this integral are developed in :
repulsion integral.
We find:
<U12> = - (4 Eo/π2) 5 π2/8
= - (5/2)Eo
Therefore:
E = 8 Eo - (5/2)Eo = (11/2) Eo =
- (11/2) 13.6 = - 74.8 eV
E = - 74.8 eV
which is close to the correct value.
4. Solutions for Helium with shielding effect:
Now, we want to bring more accuracy to our above result.
We will take into account the shielding effect, that
is an electron partially shields the nuclear charge
from the other.
Using Slater's rule, we have
for an 1s electron 0.30 for the value of the shielding constant
.
The effective charge Zeff =
2 - 0.30 = 1.70 instead of Z = 2.
By substituting 8 Eo to 2 Zeff2 Eo,
the above result becomes:
E = 2 Zeff2 Eo =
( 2 ) (1.70 2) (- 13.6 eV) = - 78.61 eV
E = - 78.61 eV
which is more close to the correct value..
|
|