1.The first law
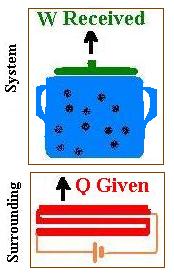 The system is the pot containing water closed by the lip. This system is placed
on its surrounding which is the burner of a stove that is a resistive heating element
connected to a battery. This element converts electrical energy to heat. The surrounding supplies
energy (heat Q) transferred to the system. The temperature of the system is increased from Ti
to Tf and the volume of water increases from Vi to Vf. This
volume expansion gives a certain work (W = ?pdV). This work W is responsible of lifting the lip.
To recap, we give heat, we receive work.
The system is the pot containing water closed by the lip. This system is placed
on its surrounding which is the burner of a stove that is a resistive heating element
connected to a battery. This element converts electrical energy to heat. The surrounding supplies
energy (heat Q) transferred to the system. The temperature of the system is increased from Ti
to Tf and the volume of water increases from Vi to Vf. This
volume expansion gives a certain work (W = ?pdV). This work W is responsible of lifting the lip.
To recap, we give heat, we receive work.
The first law of Thermodynamics states two things:
- The given heat Q is not exactly equal to the work received; a part of the heat is used
to increase temperature, say to increase the internal energy U of the system of ?U from
Ui to Uf. Forethermore, we have: Q = W + (Uf - Ui), or
Q - W = ?U = Uf - Ui
That is the the conservation of energy
- We can have any difference Q - W that can equal ?U. So the work W does not
depend on the initial or on the final state of the system, it depends on the difference between them.
This difference Q - W is equal to the net energy tranferred to the system from the surroundings. This difference is
equal to the change in the internal energy of the system ?U. The quantities
Q and W that depend on the details of the process are not variables of state for the system
but, in contrast, Ui and Uf are variables of state for the system.
The internal energy of a system is interpreted on the molecular level. Neglecting the potential
energy of interaction between molecules for an ideal or a dilute gas, the internal energy is
simply the sum of the kinetic energy of each molecule in the system. A change (increasing or
decreasing)in the internal energy for an ideal gas corresponds to a change in the kinetic energy,
therfore in temperature; for a denser gas, the potential energy between molecules contributes to
this change.
Sign conventions:
Heat Q is positive if energy is added to the system from its surroundings and negative if it is
extracted from the system.
Work W done by the system is positive if energy is transferred to
the surroundings and negative if energy is transferred to the system from the surroundings.
2. Applications
2.1 Isochoric process
Process in wchich the volume remains fixed. In this case, dV = 0, then W = 0, that is
no work is done by the system. Therfore Q = ?U which means the heat added to the system
is used to change the internal energy.
2.2 Adiabatic process
Process in wchich no energy is added from the surroundings. In this case Q = ), then
?U = - W
This kind of process occurs when the system is insulated with adiabatic walls or in the case that
of the process is performed rapidly so that the amount of heat transferred is negligible.
(adiabatic compression in an automobile engine).
2.3 Isobaric process
Process in wchich the pression remains constant. The infinitesimal stage is written as:
dW = pdV, then W = pi ?V. pi is the constant pressure.
2.4 Isothermal process
Process in wchich the temperature remains constant, that is the internal energy remains constant.
Therefore Q = W . All the amout of heat is converted to work. This kind of process occurs during the
phase change because at this point no temperature, then no internal energy is changing.
2.5 Cyclic process
Process in wchich the initial internal energy Uireturns back to the final internal energy
Uf; (the system is returned to the same state from which it started). Therfore ?U = 0; then: Q = W.
2.6 Free expansion
When initially a gas is is present in a half of a container, the other half is vacuum, connecting
the two parts lead to the free expansion of the gas in order to occupy the entire region. The system ( gas)
changes from the initial sate (Pi, Vi, Ti,Ui) to the final state
(Pf, Vf,Tf, Uf)
As the container is insolated and no work is done ( to the surroundings) then Q = W = 0 . Therefore ?U = 0
or Ui = U f. For a real gas, the temperature changes slightly; for a dilue gas or ideal
gas, the temperature does not change. In this case, so the internal energy depends only in the temperature.