1.The heat engine: Q → W
 In this heat engine, heat Q is added to the system and
work W is done by the system. This transformation of
energy Q → W can be in the opposite sense W → Q
which is the process we find when friction occurs or in a
refrigerator.
In this heat engine, heat Q is added to the system and
work W is done by the system. This transformation of
energy Q → W can be in the opposite sense W → Q
which is the process we find when friction occurs or in a
refrigerator.
The temperature is the same as the surroundings, the gas expands isothermally
as the volume of the gas increases, it does work on the turbine. The gas is
ideal and the process is isotherm then &the temperature remains constant in
the boiler.
During a cycle, then Delta;U = 0, W = QH - QC according
to the first law. QH - QC represents the net heat added to the
system during the entire cycle.
If QC is null, that is no heat is exhausted, the gas after work will
return to the boiler with no need to heat again. Therefore the gas remains stationnary
in the circuit.
But we need to have a cycle in order make the engine morking for a long time, that is
to compress again the liquid at low pressure. The solution consistts of setting a
condensor between the turbine and the pump. In this case, heat is extracted from the
working substance toward the environment. Once the liquid arrives in the boiler, it
is heated again, then compressed and the cycle is maintained. The engine works.
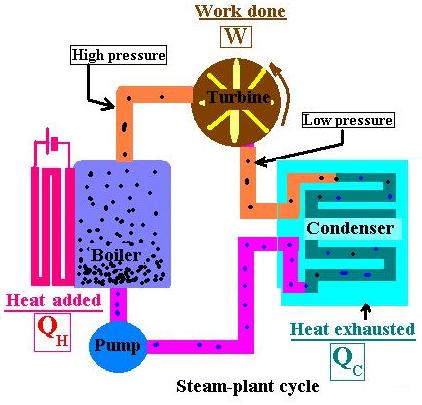
Here are the four steps:
- The boiler heat water until evaporation. Its temperature increases, so is its pressure,
- At a certain point, the vapor expands and does work by hitting the turbine blades,
- The vapor, at lower pressure, travels through the circuit and heat is exhausted from the condenser,
- The pump absorbs the liquid into the high-pressure boiler and the cycle starts again.
Heat was converted to work. This is how it operates the device called heat engine
The efficiency of the engine is defined as the ratio of the input energy to the
output energy for each cycle; that is:
η = W/QH = 1 - QC/QH
In general, the efficienty is equal at most to 40% ( 60% of heat is
wasted). There is no 100% engine; that is QC = 0 and η = 1. All the attempts
for this goal have failed.
There exists no cycle which extracts heat from a
reservoir at a single temperature and
completly converts it into work.
That is the Kelvin-Planck statement of the second law
of Thermodynamics. In other words, It is impossible to have a cycle with no heat exhausted at
low temperature. Recall that we need low temperature at the end of the cycle in order to restart
the cycle.
2.The refrigerator: W → Q
We have four major parts:
- an evaporator,
- a compressor,
- a condenser, and
- an expansion valve.
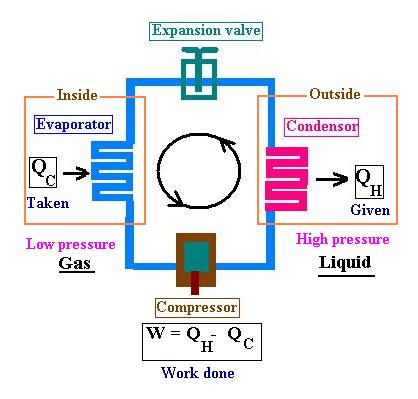
In the evaporator section, a refrigerant (the working substance) is colder that
the inside of the refrigerator, then It extracts heat from it and becomes vaporized;
changing its phase from liquid to gas.
During the next stage, a compressor(electric motor running a small piston)
compresses (pressurizes)the refrigerant vapor which becomes liquid.
This heated and pressurized liquid refregirant travels through the condenser (coil
outside the refrigerator)and gives off a part of its heat absorbed by the air around it.
Its temperature decreases.
The liquid flows through the expansion valve (using the throlling process). After passing
this valve, the pressure and the temperature of the refrigerant is reduced. The liquid
will reach the evaporator at a certain temperature.
If this temperature is not reached yet, the cycle will continue until it is done.
We define the coefficient of performance for a refrigerator as the ration
of heat extracted from the inside to the work done (by the electric motor of the
compressor; that is:
κ = QC/W
The heat pump like refrigerator as well as a freezer or an air conditioner is a system that
transfers heat from the evaporator section (inside the refrigerator), to the
condenser section (outside the refrigerator), by pumping (compressing) the
refrigerant continuously through the circuit. The system is used as cooling, but in the
reverse, it could be used as heating relatively to outside or inside space.
Using a thermocouple (thermometer), when the desired temperature is reached,
the Compressor (or pump) stops and so does the heat transfer.
To maintain -20 F (-29 C), as with a frozen food freezer, Freon-12 must
maintain a pressure of 15.3 pounds per square inch in the evaporator section.
Currently, we use the Ammonia (NH3)for the working substance instead of the
Chloro-Flouro- Carbon or CFC (brand name: Freon ). It boils at - 6.5 C (about 20 F).