Nuclear Physics
The nucleus
Radioactivity
Applications
Particle accelerators
© The scientific sentence. 2010
|
Nuclear Physics &
Particle Physics
Particle accelerators
Mass spectrometers
Mass spectrometer
A spectrometer is an apparatus to measure a spectrum; that is a graph that
shows intensity as a function of frequency, or of mass.
The mass spectrometer is an analytical instrument. It was developed in the early part
of the last century to determine the comparative masses of ionised
atoms. Later, it is used to identify the amount and type of chemicals present in a
sample by measuring the mass-to-charge ratio, and to determine the relative abundances of isotopes
as well.
The mass spectrometer works using the principles of the particle accelerators. The
simplified diagram below shows the main parts of a mass spectrometer.
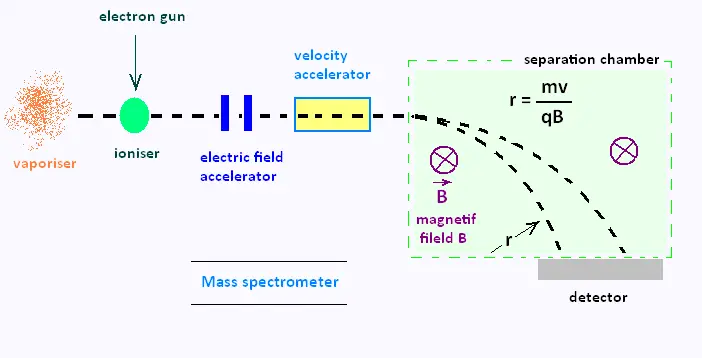
The device vaporises a sample under study. the sample is then ionised with a beam of electrons.
The formed ions are then accelerated by an electric field and pass through a velocity selector
to ensure that they are all moving at the same velocity. In the separation chamber, the ions
are subjected to a magnetic field B acting into the plane of the diagram. This field forces
the ions into circular paths of radius r given by le formula:
r = mv/qB
Where m is the mass of the ion, q is its charge, B is the magnetic field strength, r is the radius of the ion's path and v is the ion's velocity.
In practise the ions are all given the same charge, and as the velocity selector ensures that
they all move with same speed , in the same magnetic field, the radius of the ion's path is
proportional to its mass.
If, for example, the source contained the two neon isotopes 22Ne and 20Ne, two signals
would be detected. The signal from the 20Ne would be closer to the source than the 22Ne.
The mass spectrometer is similar to the LINAC in the way that it accelerates ions. It is also
similar to the cyclotron in its used of a magnetic field to produce a circular orbit for the
charged particles but of course the particles do not complete any orbits or gain any energy
in the separation chamber.

J. J. Thomson (1856 - 1940), the father of mass spectrometry, was an English physicist
at Cambridge University.
By using the first «mass spectrometer», he discovered the electron, measured its m/z, and was the
first to succeed in differing positive particles according to their m/z values. mis the mass, and z is the
charge of the related particle.
|
|