Contents
© The scientific sentence. 2010
| Bohr atom revisited
1. Potential energy of the electron
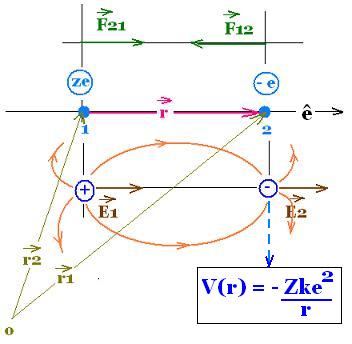
The coulomb force exerted by 1 (Z potons of charge +Ze) on 2
(electron of charge -e) is F12. Its expression is the following
Coulomb force:
F21 = F12 = (1/4πε0) q1 q2/ r2 ê
F12 is the force exerted by 1 on 2, that is rhe force exerted
by the nucleus(Z protons) on the electron.
With 1/4πε0 = k = 8.987 x 10 9 N.m2/C2, q1 = +Ze and q2 = -e,
we obtain:
F12 = - Ze2k/r2ê
(r1 - r2 = r = |r| ê, ê is the unit vector with the
line from the charge 1 to the charge 2).
The related electric field is E2 = Ee = F12/q2 = F12/(-e)
= + Zek/r2ê
Since F12 = - ∇V2 = - dV2(r)/dr, where V2 (r) is the
related electric potential,
we can write:
V2(r) = - ∫ F12 = - ∫ - Ze2k/r2ê dr =
+ Ze2k ∫ 1/r2 dr = - Ze2k/r + cst.
We choose at infinity (∞) the potential energy is
zero, therefore cst = 0, and
V2(r) = - Ze2k/r
On the electron in atom:
Coulomb force: F12 = Fc = - Zke2/r2 ê
Electric field: E2 = + Zke/r2 ê
Potential energy: V(r) = - Zke2/r
k = 1/4πε0 = 8.987 x 10 9 N.m2/C2
2. Bohr's atom
2.1. Bohr's radius atom
The Coulomb force provides the centripetal
force required for the electron to keep it moving in
the circular path.
The electrostatic force and the centripetal force are aligned,
of opposite direction and equal in magnitude. Therefore:
F12 = mv2/r
Ze2k/r2 = mv2/r
Thus:
Ze2k/r = mv2
The quantification of the linear momentum of the
orbiting electron is written as:
L = mvr = n ℏ
Combinig the two ferm relations yields:
(Ze2k/r2)(r) = mv2 = m n2 ℏ2/ m2 r2
Ze2k/r = n2 ℏ2/ m r2
Therefore:
Ze2k = n2 ℏ2/ m r
r = (ℏ2/m e2 k) n2 /Z
ℏ = 1.05 x 10-34 J s
k = 8.987 x 10 9 N.m2/C2
e = 1.60 x 10-19 C
m = 9.11 x 10-31kg
The bohr radius a0 =
ℏ2/me2 k =
(1.05 x 10-34)2/ 9.11 x 10-31 (1.60 x 10-19)2 8.987 x 10 9 =
0.0053 x 10-68 + 31 + 38 -9 = 0.53 x 10-10 m = 0.53 Angstrom.
Then:
r = a0 n2 /Z
For the hydrogen atom, n = 1, and Z = 1, then:
r = a0 = 0.5 Angstrom.
2.2. Bohr's energies atom
The total energy is E = KE + PE
KE = (1/2)mv2
PE = - Ze2k/r
From the equality F(Coulomb) = F(Centripetal)
Ze2k/r2 = mv2/r, we have:
- PE = 2 KE , so:
E = KE - 2KE = - KE = + EP/2 = - Ze2k/2r =
Using the expression of r:
r = (ℏ2/m e2 k) n2 /Z
We find:
E = - Ze2k . m e2 k/2ℏ2 Z /n2 = - me4k2/2ℏ2 (Z2/n2)
E = - me4k2/2ℏ2 (Z2/n2)
ℏ = 1.05 x 10-34 J s
k = k = 8.987 x 10 9 N.m2/C2
e = 1.60 x 10-19 C
m = 9.11 x 10-31 kg
me4k2/2ℏ2 = 9.11 . (1.60)4 . 8.9872/2. (1.05)2
= 2183.44 x 10-31 -76 + 18 + 68 Joules =
2183.44 x 10-21 Joules = 1364.65 x 10-21+19eV =
13.6 eV
Therefore:
E = - 13.6 (Z2/n2) eV
2.3. Speed of the electron in the atom
The energy of the electron is entirely kinetic. So,
E = - KE = - (1/2) mv2 = - 13.6 Z2/n2
Let Eo = 13.6 eV. Therefore:
v2 = (2 x Eo/m) Z2/n2
v = (2Eo/m)1/2 Z/n
Eo = 13.6 eV = 13.6 x 1,6 x 10-19 J
v = (2 x 13.6 x 1.6 x 10-19/9.11 x 10-31)1/2 Z/n
v (electron) = 2.2 x 106 Z/n (m/s)
|
|