Contents
© The scientific sentence. 2010
|
X-Rays
1. Production
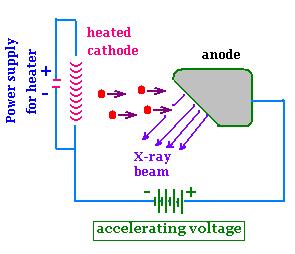
In a tube, from a heated cathode, electrons emitted move to and strike a
target metal anode. These electrons are accelerated
through a potential difference of the order of 103
106 103. As a result X-rays scatter from
the anode. They were first produced in 1895 by Wilhelm Rontgen
(1845-1923). The tube is evacuated allowing electrons to move
without colliding with air molecules. Typical produced X-ray
wavelengths are 10- 3 to 1 nm. When interacting
with matter, x-rays are highly penetrating rays. They are
ionizing radiation that can produce physiological
effects such as mutations or cancer in tissue. They are
mainly used in medical facilities to image some parts of
body such as bones.
2. X-Rays are photons
X-Rays are electromagnetic waves with high frequencies. They are produced in two ways:
by electrons during their deceleration and by electrons during transition within an atom. Both X-rays are produced on impact with the target:
1- When electrons (charged particles) are suddenly decelerated (as in the tube), they
produce X-Rays. The produced rays are called bremsstrahlung radiation (from German braking radiation).
2. When electrons make transitions in heavy elements,
generally between lower atomic energy levels, they produce X-Rays.
X-rays produced in this way have definite energies which is
determined by the atomic energy levels.
They are called characteristic X-rays
An ejected electron from the inner shell of the atom
of the metal target,leave a vacancy to be filled by an electron
dropping down from higher energy level.
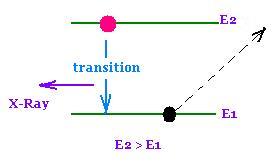
The bombarding electrons with enough energy can produce X-Rays by bremsstrahlung and by transition thereafter.
3. Example
In the process of producing X-Rays, the
kinetic energy KE = (1/2)mv2 (velocity v and
mass m) of an electron projectile, which is equal
to its total energy E, is transformed to produce a bremsstrahlung
X-ray photon on impact with the target atom. If the entire energy is used,
then an X-ray of maximum energy , then maximum frequency, and
minimum wavelength is produced. What are (a) the speed of the electron and
(b) the value of the X-Ray's wavelength if the bombarding electron
is accelerated under 10.0 kV?
a)
E = KE = KE = (1/2)mv2 = e V = 1,6 x 10- 19 10,000.00 (Joules)
= 1,6 x 10- 15 J
v = [2 1,6 x 10 - 15 / 9.1 x 10 - 31 ]1/2 =
6 x 107 m/s
b)
E = h ν = 1,6 x 10- 15 J
ν = E/h = 1,6 x 10- 15/6.626068 x 10- 34 = 0.24 x 1019 m
ν = c/λ
λ = c/ν = 3 x 108 /0.24 x 1019
= 1.25 x 10- 10 m = 0.125 nm
More quickly:
λ = hc/eV = 4.14 x 10- 15 (eV.s) 3 x 108 (m/s)/
e 10.0 x 103 (V) =
4.14 x 10- 15 3 x 108 m /
10.0 x 103 = 0.125 nm
4. X-Rays spectra
The bremsstrahlung process do not depend on the target material. It depends only
on the accelerating voltage. Whereas the characteristic X-ray process do depend
on the target material. Every spectrum of X-Rays that stem from an element
is characteristic of this element, because each element has its proper and
unique set of atomic energy levels.
The involved element to produce characteristic X-Rays spectra are essentially
the heavy elements and within their inner shells, which the related energy levels
are about 100 to 1000 eV, rather than the light elements or the outer
electrons in heavy elements that involve few eV and responsible for optical or visible
spectra as Balmer's series or the others in Hydrogen atom.
The spectrum of an X-ray contains two parts: The bremsstrahlung part depending on the
accelerating voltage; and
the characteristic part depending on the target element. In the following figure, we have the intensity of
the produced X-ray versus its wavelength for the molybdenum element target.
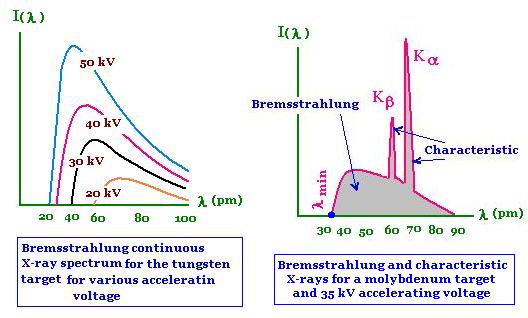
The obtained spectra shows the continuous spectrum corresponding
to the bremsstrahlung and the two peaks Kα and Kβ
corresponding to characteristic X-rays.
The minimum wavelength corresponding to the bremsstrahlung is:
λmin = hc/e Vacc
Vacc is the accelerating voltage.
The spectrum Kα has the wavelength:
λKα = h c/(EL - EK)
The spectrum Kβ has the wavelength:
λK β = h c/(EM - EK)
5. Moseley's law
Using X-ray diffraction technique, in 193, Moseley, studied in details
these spectra. He found that the spectrum Kα line (the most
intense wavelength line in the characteristic X-rays spectrum) for
a particular element varied smoothly with the atomic number Z of that element.
That is not the case for optical spectra that vary greatly for elements
with adjacent Z values.
Moseley found an empirical formula that express
the frequency ƒ of an X-ray for Kα line versus the
atomic number Z of the element. It is :
ƒ = 2.48 x 1015 (Z - 1)2 (Hz)
plotted generally as
ƒ1/2 x 10-8 (Hz1/2) versus Z.
Moseley's law
ƒ = 2.48 x 1015 (Z - 1)2 (Hz)
|
|