AUGER effect
Let's consider this figure:
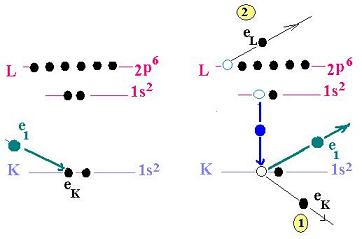
This process shows the mechanism of the Auger electrons.
Generaly, the mecanism of ionizing an atom ejecting an electron
from a inner shell and leading to an emission of a second electron
from a higher shell, is called the Auger process.
Consider a medium bombarded with a beam of rays or
particles. Commonly, we use electrons as incident beam.
The steps of the process are the following:
- A primary incoming electron irradiates the medium.
- The target atom becomes ionized by loosing an electron.
- The porpose is at first to remove an inner shell electron
in order to form an inner shell vacancy.
- The related atom becomes excited by loosing an electron.
- The atom relaxes back to a lower energy state. An electron
( from the atom ) leaves a higher shell with a higher bounding
energy, fills the vacancy and releases the excess energy.
- This excess energy allows another electron to escape the atom.
This is the Auger electron. It carries off this excess energy
in kinetic
energy form. This Auger electron is ejected from a higher
energy level.
- In the final state, the atom, devoid of its proper
two electrons, becomes
doubly-ionized atom.
Pierre Auger, in 1923, discovered the mechanism while irradiating
samples with X-rays.
The figure above is related to low atomic number elements.
For the higheratomic number elements, the most probable
transitions L-M and M-N.
The ionization of the related atoms requires a beam of high energy
electrons in the range 2 - 10 keV.
When using soft X-rays of energy in the range 1000 - 2000 eV ,
we talk about the XAES
Now, The Auger Electron Spectroscopy (or AES) is a technique
used to determine the composition of the surface layers fo a sample
and thin-films.
The energy inolved in this mechanism is as folow:
To liberate the Auger electron, the remaini energy
must first overcome its binding energy: Eb
If Ep is the incident electron, Exy, the transition (relaxation)
energy ( from x to y) and Ee the remainder feaction, tha is the
kinetic energy of the
Auger electron, we can write:
Ep = Exy + Eb + Ee (1)
Hence:
Ee = Ep - (Exy + Eb) (2)
Auger spectroscopy is based on the measurement of this energy.
Knowing Ep, Ee must be determined in order to have
information about Eab, that is the analysis of the emitted
Auger electron.
In the step number 5, instead of having Auger
electron, we can get the X-ray fluorescence mechanism.
|

