Zeeman effect
The angular momentum L of the electron (e-) of mass m moving at
the velocity v around the proton is given by:
L = r x P
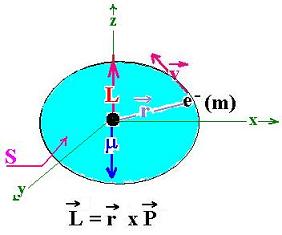
P is the linear momentum of the electron over the
circumference, that is mv.
r is the distance of the e- with respect to the origine ( the proton)
The magnetic moment of the electron is given by this expression:
μ = I S
Where I is the related current. We can evaluate its expression by:
I = -e/T ( T is the period of the revolution)
T = 2πr/v
and S = πr2
Then:
μ = (-e v /2πr)πr2 = -e v r /2 = -e L/2m
Where L = mvr
Rewrite then this magnetic momentum:
μ = - e L/2m
Let's remark that the magnetic momentum gathers the
own features of the system, that is witout any exterior
intervention.
Now; let's apply a magnetic field B to the system.
Exactly as a spinning top, this system will precess
as shown in the following figure:
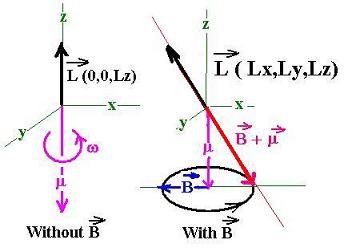
This shift in the magnetic momentum in direction, not in
magnitude, is the cause of the splitting in the energy
levels of the electron in its orbit.
From the Schrodinger equation related to this system
(the hydrogen atom), we know, without the spin part,
that the electron occupies one orbit (state) specified by
three numbers:
- Principal quantum number: n
- Orbital quantum number: l
- Magnetic quantum number: m
The shell n is completed with 2 n2 electrons
( 2 for the first shell; 8 for the second: ans so forth). this
number gives the energy levels of each electron over its orbit.
Given an n, then l takes n values: 0, 1, 2, ...; n-1.
The number l is directly related to the angular momentum.
If an external magnetic field is applied for the system,
then each l will split into 2l+1 values of
m: -l, ..., -2, -1, 0, +1, +2, ...+l.
This effect was first observed in 1896 by Pieter Zeeman working
on the sodium in a strong magnetic poles.
It's obvious that if l = 0 ( mainly for n = 1), the splitting
will not occur.
This example shows the splitting related to the 2p6
sbshell, for n = 2 : l=1 : m = -1,0,+1:
|

