Contents
The nucleus
Radioactivity
Radiation Measurements
Black body radiation
Statistical Mechanics
Radiation and scattering
Related topics
Related links
© The scientific sentence. 2010
|
Moseley's law
1. Characteristic x rays
When an electron falls from L shell to K shell, the photon (electromagnetic radiation)
produced is called the Kα ray. If the process is from M to K ,
the ray is Kβ and son on ..
Electrons with lower values of n are more bounded to the nucleus
than those with higher values.
The shells correponding to the stationary states are given letter names:
the n = 1 shell is called K shell, n = 2, the L shell, and so on.
If enough energy is transferred to a K electron to dislodge it from the
atom, the atom will be left with a vacancy in its K shell. The atom is
most stable in its ground state, another electron from one of the higher
shells will fill the inner-shell vacancy at lower energy emitting electromagentic
radiation. We call this an x ray. This x ray carries the energy :
EK = E1 - E2,3, ...
This emitted x ray is known also as the characteristic x-ray
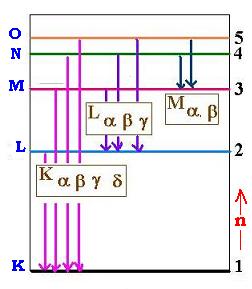
For each element, the energy levels are different, so the characteristic K x-rays are different.
2. Empirical Moseley's law
In 1913, H.G.J. Moseley, working in Rutherford's Manchester laboratory, was engaged in cataloguing the characteristic x-ray
spectra of series of elements. He concentrated on the K shell and the L shell x rays produced in an x-rays tube.
By comparing frequencies, Moseley found the following empirical relationship, extracted from his plot Z versus the
square root of the meaured frequency:
νKα = (3cR/4)(Z - 1)2
Where : Z is the atomic number , c is the speed of light, and R is the Rydberg constant.
3. Moseley's law from Bohr's atom
Supposing that there are two electrons in the K shell. For an electron L shell, to fill the missing K shell electron, it feels
an effective charge (Z - 1) (+ Ze due to the proton and - e due to the remainig K shell electron). Thus the
transition between L and K shell (n = 2 → n = 1) involves a photon of wavelength :
1/λKα = R (Z - 1)2 [1/12 - 1/22] = (3R/4)(Z - 1)2
Wich is the same formula found empirically above.
|