Contents
The nucleus
Radioactivity
Radiation Measurements
Black body radiation
Statistical Mechanics
Radiation and scattering
Related topics
Related links
© The scientific sentence. 2010
|
Attenuation coefficients
1. Expression of the attenuated intensity
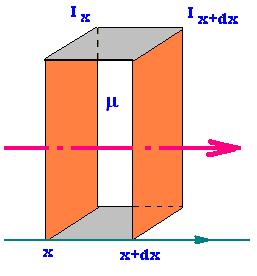
When a narrow beam of mono-energetic photons, such as x-ray, γ rays,
penetrates a surface of a material; it experiences a change in its intensity,
owing to absorption or scattering that occur during the travel of the beam
accross the material. The expression of the intensity of the beam the long
of an axis parallel to the direction that photons beam can be calculated as:
dIx = Ix+dx - Ix = - (Ix - Ix+dx) = - coefficient dx Ix
Or:
dIx/Ix = - coefficient Ix dx.
Integrated with respect to the thickness of material traversed "dx", and
letting coefficient = μ gives:
ln I(x) = - μ x + constant → I(x) = Constant e - μ x
At x = 0, I(o) = Io; then, at the traveled distance "x", we have
I(x) = Io e - μ x
2. Linear attenuation coefficient
 The coefficient μ must have the dimension of length-1.
It is called the linear attenuation coefficient. It is generally
expressed in cm-1.
The coefficient μ must have the dimension of length-1.
It is called the linear attenuation coefficient. It is generally
expressed in cm-1.
The linear attenuation coefficient μ is defined as the fraction of an
incident beam of photons that is absorbed or scattered per unit thickness
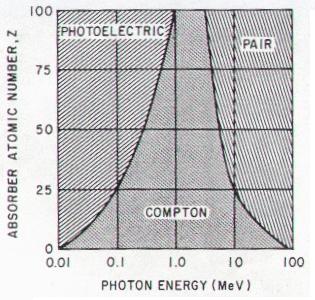
of the target absorber. The photoelectric effect dominates at low
photon energies (< 26 keV) in soft tissue. With higher energy photons at
low Z materials, Compton scattering dominates. Rayleigh scattering comprises about
10% of the interactions in mammography and 5% in chest radiography.
The average distance traveled by a photon between two successive interactions
is called Mean Free Path (MFP), and can be calculated from linear attenuation
coefficient: λ = 1/μ.
3. Mass attenuation coefficient
The coefficient μ is used as μ/ρ and called mass attenuation coefficient,
where ρ is the density of the target material. It is usually expressed in units
of cm2/g. It is obtained by measuring the values of Io, I and the
thickness "x".
4. Total mass attenuation coefficient
The mass attenuation coefficient is due to absorption coefficient related to the
incoherent processes, as the phoelectric effect, Compton effect and pair
production; and scattering coefficient due to coherent effect as Rayleigh
scattering processes. We write:
μ/ρ (Total) = μ/ρ(absorption) + μ/ρ(scattering), that is:
μ/ρ (Total) = μ/ρ(Photoelectric) + μ/ρ(Pair-Production) +
μ/ρ (coherent:Rayleigh) + μ/ρ(incoherent:Compton)
For mixtures and compounds, the values of the mass attenuation coefficients
are obtained by additivity: μ/ρ = ∑ wi (μ/ρ)i,
where wi is the fraction by weight of the ith atomic constituent.
5. Total related cross section
The cross section that an incident photon makes an effect (absorption or
scattering) within the target material is the total cross section due
to the attenuation effects; that is the absorption effect and the scattered effect.
σ (Total) = σ(absorption) + σ(scattering), that is:
σ (Total) = σ(Photoelectric) + σ(Pair-Production) +
σ(coherent:Rayleigh) + σ(incoherent:Compton)
6. Relationship: μ/ρ and σ
If S is the right area perpendicular to the path of the
incident beam of photons through the material, the related
volume is V = S dx. Per atom, it is equal to S dx/N, where N
is the number of atoms within the volume V. The volume can be
expressed as V = m/ρ where m and ρ are respectively
the mass and the density of of the volume V of the material.
Hence, per atom, we have the surface Sa = V/dx = ma/ρdx.
Per unit length, the surface Sa is called the cross section
that the incident photon makes an effect with the target atom and denoted
σ. Therfore dx = 1/μ.We have then ρ = maμ/σ.
With ma = u A/Na (u is the atomic mass unit, A is the
relative atomic mass of the target element, and Na the
Avogadro number), the product μ/ρ takes then the following form:
μ/ρ = σ Na/uA.
μ/ρ = σNa/uA [per atom and per unit of thickness]
Where:
σ is the cross section per electron (the probability that the incident photon makes
an effect in the target element),
ρ the density of the material target,
Na is the Avogadro number (6.023 23/mol),
u is the atomic mass unit (mass of an atom of nuclide 12C/12), and
A is the relative atomic mass of the target element.
7. Mass energy transfer coefficient
The mass energy transfer coefficient is the product of the mass attenuation
coefficient and the fraction of energy transferred to the charged particles as kinetic
energy, by the interacting incident photons. That is the fraction "ƒ" of the mass attenuation
coefficient that produces kinetic energy forejected electrons from their atomic shell.
It is written as: μtr/ρ. We have:
μtr/ρ = Na/uA x {ƒphσ(Photoelectric) +
ƒppσ(Pair-Production) + ƒcohσ(coherent)}
where the low contribution of the σ(incoherent:Compton) transfert is neglected.
ƒx is the related process fraction.
For mixtures and compounds, the values of the energy transfert coefficients
are obtained by additivity: μtr/ρ = ∑ wi (μtr/ρ)i , where wi is the
weight of the ith atomic constituent.
8. Example of the mass attenuation coefficients graph
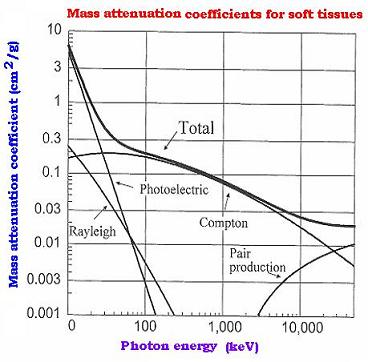
|