Contents
The nucleus
Radioactivity
Radiation Measurements
Black body radiation
Statistical Mechanics
Radiation and scattering
Related topics
Related links
© The scientific sentence. 2010
|
Nuclear fission and fusion processes
Radioactivity is a nuclear spontanous reaction without any intervention.
Other nuclear reactions such as fission and fusion are artificial.
The fission process is the splitting of a nucleus to form two smaller
ones, while fusion is the fusing of two smaller atoms to form a larger one.
1. Fission:
Fission is concerned by heavy nuclei. In such process, a nucleus, with large
number of nucleons, captures one neutron, and splits into two fragments with
smaller number of nuleons, followed by the emission of one or more neutrons. The
two obtained fragments have a low binding energy per nucleon; then they are
more stable regarding the initial nucleus. The fission process deals essentially
with U(235,92), U(233,92) Uranium atoms, and P(239,94) Plutonium atom.
The neutron (without electric charge) approches a nucleus without being deviated
from its direction by electrostatic interactions. Those that have low kinetic
energy (≈ 0.1 eV corresponding to the energy of a neutrons at ambient
temperature), are more likely to produce the 235-Uranium nucleus fission. They
are called thermal neutrons. Fission is a nuclear process which requires
incident neutrons to start the reaction. Each produced neutron will produce one
or more others; therefore nuclear chain reaction will takes place. This process
does not last for a long time. It liberates a strong quantity of energy in a
very shorttime that is an explosion such as in an atomic bomb (bomb A). In nuclear
reactors, the fission process, escially the neutron emission rate, is controlled.
The reaction for one atom 235-Uranium is the following:
U(235,92) + n → Xe(139,54) + Sr(95,38) + 2n.
The number of charge is always conserved. The energy liberated by the
reation is ε = (mi - mf) c2
We have for the individual masses:
U(235,92): mU = 234.993 uma
Xe(139,54): mXe = 138.914 uma
Sr(95,38): mSr = 94.856 uma
n: mn = 1.009 uma
Then:
mi = mU + mn
mf = mXe + mSr + 2mn
mi - mf = mU - mXe - mSr - mf = 0.215 uma
(about 0.1% of the initial 235-Uranium is transformed in energy).
Then: ε ≈ 0.215 x 1.66 x 10 -27 x 9 x 1016 ≈ 3.2 x 10 -11 joules.
This energy is find in most part as kinetic energy for the produced nucleus,
the rest is taken by the neutrons and γ rays.
For one mole, we have: E = Na ε = 6.023 x 10 23 x 3.2 x 10 -11 ≈ 2 x 1013joules, which is an enourmous energy quantity!.
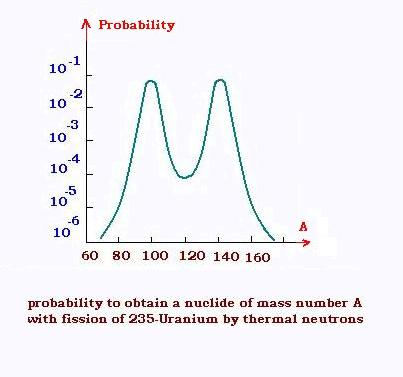
The products of the fission process are very large for a same initial nucleus. This
is due to the multiplicity of the fission modes and the decaying transformations of
the produced nucleus. For the 235Uranium, we can have also the reation
U(235,92) + n → Ba(139,56) + Kr(94,36) + 3n .
Generally, the probability to obtain nuclei by fission
looks like the 235-Uranium probability distribution profile by thermal neutrons,
shown at right. This crve is slightly modified when the captured neutrons are
more energetic. The fission of the 239-Plutonium and the 233-Uranium gives
similar results. The 239-Plutonium is an artificial element. It is produced
by the capture of a neutron of high kinetic energy (rapid neutron) of the
order of some MeV by the 238-Uranium, followed by the emission of two electrons
(β-). By an identical process, the 232-Thorium leads to
the 233-Uranium via a protactinium (Pa) by the following
series:
Th(232,90) + n → Th(233,90), then:
Th(233,90) → Pa(233,91) + e-, and:
Pa(233,91) → U(233,92) + e-
The 232-thorium nad the 238-Uranium are relativeley abundant in nature.
The 232-thorium leads to the 233-Uranium and the 238-Uranium leads to the
239-Plutonium. Both of the two products are fissile. We say that the
232-thorium and the 238-Uranium are fertile.
The Uranium in nature is composed of 0.72% of 235-Uranium fissile and
99.27% of 238-Uranium fertile.The 238-Uranium is not fissile, but it is
converted to the 239-Plutonium which is fissile as the 235-Uranium and
the 233-Uranium.
2. Fusion:
In the process of fusion, two light atoms and combine to form a heavier atom,
followed or not by emission of aparticle such as neutron, electron, positron,
proton, ... .
For example, the reaction prduced in the sun is:
H(1,1) + H(2,1) → H2(3,2)
As for a fission, a fusion reaction conserves the bumber of nucleons
and the number of charge.
The energy liberated by this reaction is: ε = (mi - mf) c2
We have for the individual masses:
H(1,1): m1 = 1.0073 uma
H(2,1): m2 = 2.0134 uma
H(3,2): m3 = 3.015 uma
Then:
mi - mf = m1 + m2 - m3 = 5.79 x 10 - 3 uma
ε ≈ 5.79 x 10 - 3 x 1.66 x 10 -27 x 9 x 1016 ≈ 8.65 x 10 -13 joules = 5.4 MeV.
The fusion is favored when the binding energy per nucleon of the final
nucleus is greatre than the ones of the initials nucleus.
For the reaction:
Li(7,3) + H(1,1) → Be(8,4),
where the binding enrgy per nucleon for the Lithium is εLi = 5.4 MeV and
for the Beryllium εB2 = 6.8 MeV; the liberated enrgy is calculated as follows:
We separate first the 7 nuclons of the Beryllium that requires the enrgy
ELi = 7 x εLi = 7 x 5.4 = 37.8 MeV.
Next, reconstitute the Be(8,4) from 8 nucluons (7 for Li(7,3) plus 1 for H(1,1)), that requires
the energy EBe = 8 x εBe = 8 x 6.8 = 54.4 MeV. Hence the
energy liberated by the reaction is: EBe - ELi = 54.4 - 37.8 = 16.6 MeV.
To realise this the initial nucleons must very energetic in order to overcome the
strong repultion electric forces between the positive charges of these nucleons.
Hence the fusion requires nucleons with high kinetic energy (≈ 1 MeV).
The temperature of a medium increases with respect to the kinetic energy of the
constituting particles; therefore, the fusion is possible only at temperature about
one billion degrees oC; that we call the thermonuclear fusion.
The fusion is spontaneous within stars. Artificially, on earth, these high temperatures
conditions could be reproduced by an explosion of a bomb A. This can triggers the
reaction:
H(2,1) + H(3,1) → He(4,2) + n ,
wich is the principle of the bomb H. For One kilogram of the initial charge of fusion
(Deuterium and Tritium combined), the energy liberated is on the order of produced by
200 kilo-tons of TNT (Trinitrotoluene).
The fusion releases much more energy per mass compared to the fission. In other words, the
energy liberated from 1kg of 235-Uranium as fuel is less than that one from the
fusion of 1kg of Deuterium and Tritium combined. Currently, the related research
focuses on obtaining high temperature (at least some tens of millions degrees) plasma
or using lasers to experiment the theortically possible fusion reaction.
|