Contents
The nucleus
Radioactivity
Radiation Measurements
Black body radiation
Statistical Mechanics
Radiation and scattering
Related topics
Related links
© The scientific sentence. 2010
|
Rayleigh scattering
1. The equation of a moving bound
electron in an electric field
Let us consider an electron bound elastically to its rest position.
When an electric field E is applied on this electron, this latter
will undergoes the electric force ( the electric component of
the Lorentz force). Let' write this force as:
fe = - e E
This force on the electron displaces it from its first position;
the electron then tends to return to it. The related restoring force
can be written as:
fr = - r s
Where s is the displacement of the electron,
and r the damping coefficient.
r = m ω02
m is the mass of the electron,
ω0 is the oscillation frequency of the electron.
As a friction in Mechanics, let's assume that the electron
undergoes a damping force; wich can be written as:
fd = d ∂s /∂t
Where d is the damping term = m g ω0
g is a constant g that is typically much less than unity.
The Second Newton law for the moving electron can be written as:
m ∂2s /∂t2 = fe + fd + fr
Then the equation of motion of the electron is expressed as the following
differential eqation:
m ∂2s /∂t2 = -e E - d ∂s /∂t - r s
This equation takes the form:
∂2s /∂t2 + gω0 ∂s/∂t + ω02 s = (-e/m) E (1)
2. Rayleigh scattering
Let us now consider the scattering of electromagnetic radiation by such electron
described above:
Let's assume that the displacement and the electric field
are dependent on time buy the form of : exp(-iωt), then:
s = s0 exp(-iωt) (2)
E = E0 exp(-iωt) (3)
The equation (1) takes the form:
- ω2 - i gωω0 + ω02 = (-e/m) E0 /s0 = (-e/m) E/s
Then s = (-e/m) E/ (- ω2 - i gωω0 + ω02) (4)
∂2s /∂t2 = - ω2 s (5)
Substituying (5) in (4), we get:
∂2s /∂t2 = - ω2 (-e/m) E/ (- ω2 - i gωω0 + ω02)
Rearanging, we obtain:
∂2s /∂t2 = ω2 (e/m) E/ (ω02 - ω2 - igωω0 ) (6)
Recall that the Thomson cross section is set witout the damping and
restoring factors and that it is proportionnal to : <Eout2>/ <Eint2>
and that <Eout2> is proportinnal to <Eint2>
Then the cross section does not depend on <Eint2>
Finaly,
σTotal/Rayleigh = σTotal/Thomson |ω2 / (ω02 - ω2 - igωω0 )|2 (7)
After calculating the module between the two||, we find:
σ(Total-Rayleigh)=
σ(Total-Thompson) x ω4/[(ω02-ω2)2+(gωω0)2] (8)
When ω = ω0, we obtain the maximum value of the cross
section, that is for resonant scattering. The scattering cross section becomes :
σTotal/Rayleigh = σTotal/Thomson (ω/gω0)2 = (1/g)2 (9)
Recall that ω and ω0 are the frequencies
of the incident light and the scattered radiation resectively.
In the case of the binding energy of the electron is
greater than the incident energy of the photon ( ω<<ω0),
the relation (8) becomes:
σTotal/Rayleigh = σTotal/Thomson (ω/ω0)4 (9)
In terms of wavelength,λ = c/2πω (9) becomes:
σTotal/Rayleigh = (λ0/ λ)4 (10)
Let's write:
σ(Total-Rayleigh) = constant x 1/λ4
Where λ is the wavelength of the incident radiation.
This formula is the scattering cross section of the incident
radiation by the medium. It depends on the inverse fourth power
of the wavelength of the incident radiation. It is known as the
Rayleigh scattering cross section.
The Rayleigh scattering is known as a coherent effect, owing
to the fact that no energy is lost acroos the target during the passage
of the photon through the target material.
From the formula related to the cross section, the similar
relation , related to the intensity can be written as:
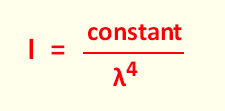
3. The blue color of the sky
These formulas are appropriate to the scattering of visible radiation
by gas molecules. In the case of the target medium is the air of the
atmosphere, this relationship gives the famous explanation of the blue
sky: the air molecules of the atmosphere scatter the shorter
wavelength components of the sunlight.
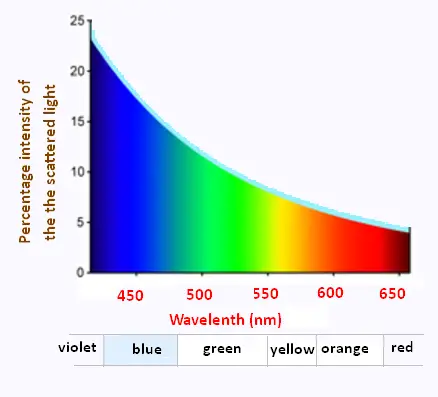
The strong wavelength dependence of the scattering (1/λ4)
means that shorter (blue) wavelengths are scattered more strongly
than longer (red) wavelengths.
4. The white color of the sky
The water droplets in the clouds are many times larger than the
wavelength of the incident light, then many shorter in terms of
the frequency (ω). In this case σTotal/Rayleigh
= σTotal/Rutherford.
That is the scattered radiation has the same frequency as the incident light.
Foretheremore, the clouds scatter entirely the received light and look white.
|