Classical Mechanics
< E > = κT
But Quantum Mechanics shows that the average energy
depends on the temperature.
< E > = < (1/2)mv2 > + < (1/2)κx2> =
(1/2)κT + (1/2)κT = κT
< E > = κT
< E > = Σ ni Ei =
N Σ Ei exp[- βEi]/Z =
(N/Z)Σ Ei exp[- βEi]= N ΣEi P(Ei)
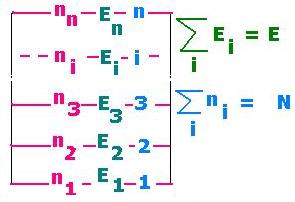
| |
"i" is the ith level
P(Ei) = exp[-βEi]/ Z
Z = iΣ0→∞ exp[-βEi] = 1/β
|
Where: P(Ei) is the probability for ni particles in the
level "i" to have the energy Ei. The sum is related to all the states "i".
The normalization of this probability can be written as:
Σ P(Ei) = 1.
or :
∫ dE P(E)= 1 [from: 0 → ∞]
∫ dE P(E) = (1/Z)∫ dE exp[- βE] =
(1/Z)[- 1/β]exp[- βE](From: 0 → ∞)= β/Z = 1
Thus:
β = 1/Z (2.)
We can write:
P(Ei) = β exp[- βE] (3.)
We have:
< E > = Σ Ei P(Ei) =
Σ Ei exp[- βEi]/Z =
(1/Z)∫ E exp[- βE]dE
∫ x exp[-β x] dx = (1/β2) ∫ y exp[- y ]dy
Where y = β x
By part;
∫ y exp[- y ]dy; = - y exp [- y] - exp [- y] = - (y + 1)exp[- y]
(form : 0 → n, n is the number of states). We take n large, then
∫ y exp[- y ]dy = [- (y + 1)exp[- y]] [: 0 → n] = 1
It follows that:
∫ x exp[-β x] dx = 1/β2, and
< E > = 1/Z . β2
From the equation (2.), we have : < E > = 1/β
From the equation (1.), we get:
< E > = 1/β = κT
Foretheremore,
β = 1/κT
The relationship (3. ) becomes:
P(Ei) = (1/κT) exp[- E/κT] (4.)
|