A sigle wave
Superposition of waves
© The scientific sentence. 2010
|
Matter-waves concept
1. The De Broglie relationship
By 1920, Physicists needed to improve the
Bohr's atom model. Prince Louis de Broglie made a step to this matter. In 1924, he made a spectacular hypothesis.
Just observing E2 = p2c2 + m02c4 (1.1), and
p = E/c = hν/c = h/λ( m0 = 0) (1.2)
for a photon which has a wavelength, he extended the notion of wave to any particle.
The equation (1.2) gives:
λ = h/p
p is the momentum, λ is the wavelength, and h is the Panck's constant.
Any corpuscle has both wave and particle properties. Wave is needed to explain interference and diffraction
and particle for compton and electric effects. We talk about Matter waves.
Obviously, we cannot experiment the duality relation of De Broglie for heavy objects as a
car, but it can be done for light particles as
electrons.
2. About Bohr' quantization condition
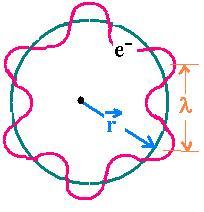 The electron is matter: L = mvr = pr (2.1)
The electron is matter: L = mvr = pr (2.1)
The electron is a wave: nλ = 2πr (2.2)
Substituting (2.2) in (2.1) and using De Broglie relationship, one obtains:
L = p (nλ/2π) = (h/λ)(nλ/2π) = nh/2π.
That is the Bohr's assumption to quantize the angular momentum.
See the related
©: The scientificsentence.net. 2007.
|
|
|